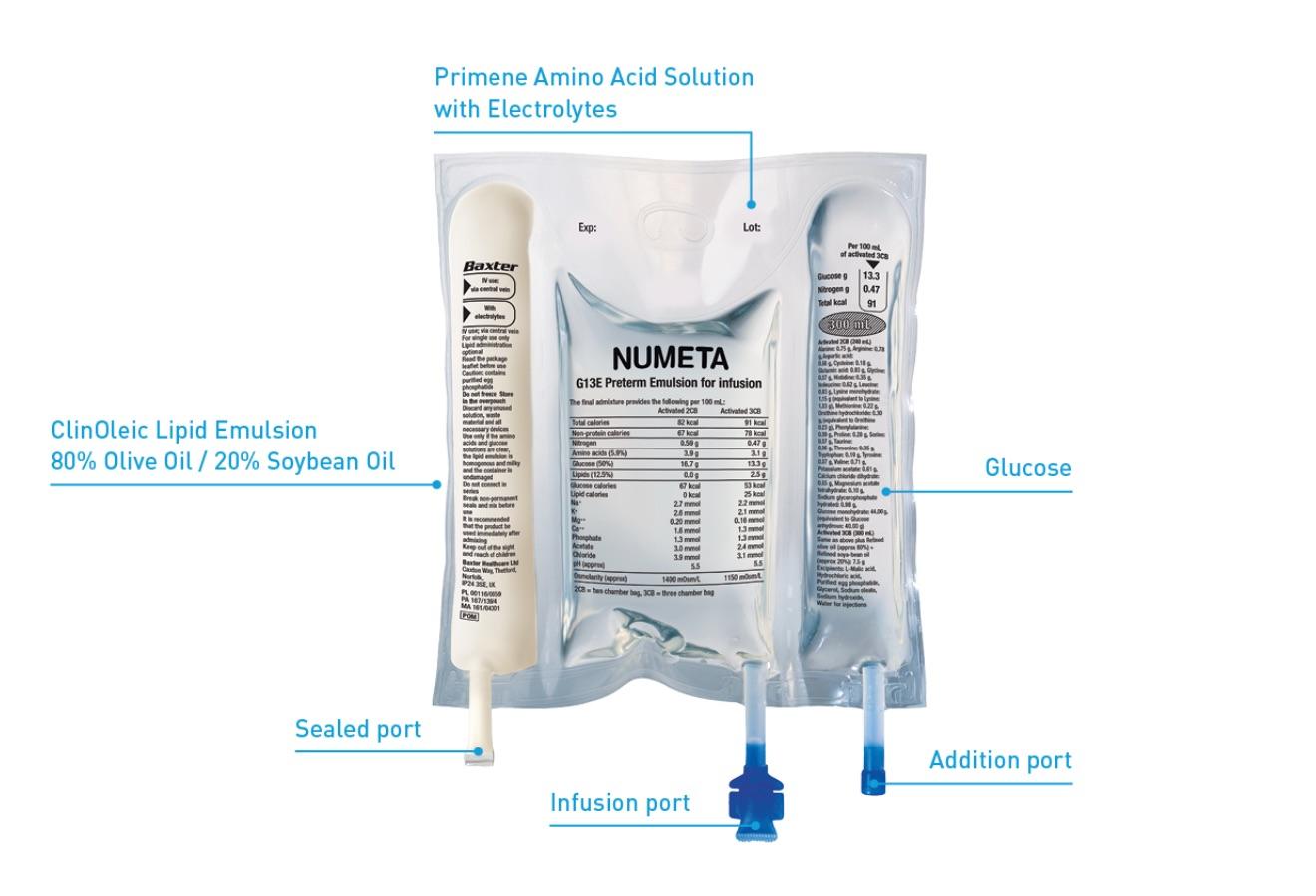
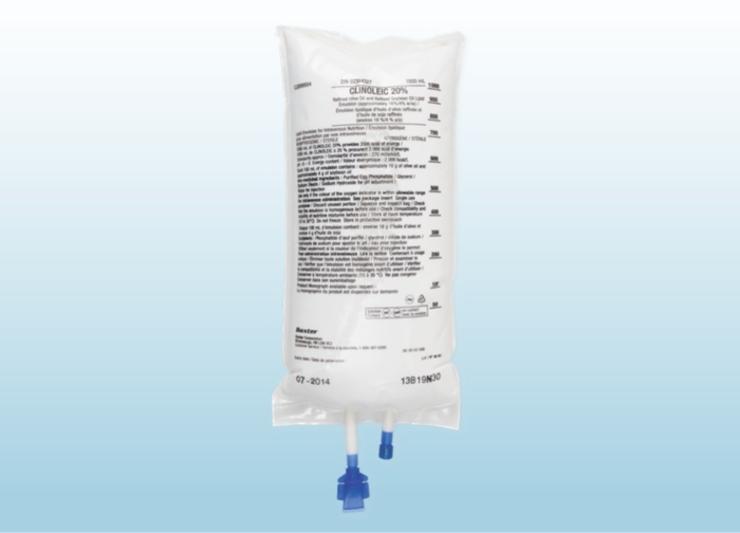
Numeta
(glucose solution, lipid emulsion, amino acids, electrolytes)
The first and only licensed ready-to-use three-chamber bag parenteral nutrition (PN) solution specifically designed for neonatal and paediatric patients.

When oral or enteral nutrition is not possible, insufficient or contraindicated, Numeta provides neonatal and paediatric patients specifically formulated nutritional support that could help reduce growth deficits and improve long-term growth outcomes.1-4 Numeta PN bags are the first and only ready-to-use three-chamber bag (3CB) solutions for preterm neonates (Numeta G13%E Preterm), term infants and children up to 2 years (Numeta G16%E ), and children older than 2 years through adolescence (Numeta G19%E ).1-3 They are designed to give the flexibility of administering PN with or without lipids and the ability to add micronutrients and compatible additives as needed.1-3 Numeta may help reduce the risk of compounding errors, bloodstream infections, and medication errors that are seen with compounded PN.4-5
Parenteral Nutrition is a complicated, yet critical, therapy for many preterm infants and paediatric patients
Poor nutrition in preterm infants and critically ill children is associated with higher mortality and can be detrimental for long-term growth.6, 7 For patients who cannot receive adequate nutrition orally or enterally, PN can be lifesaving.7 Because of its complexity, PN is classified as a “high-alert” medication with an increased risk of causing significant patient harm if provided incorrectly.8
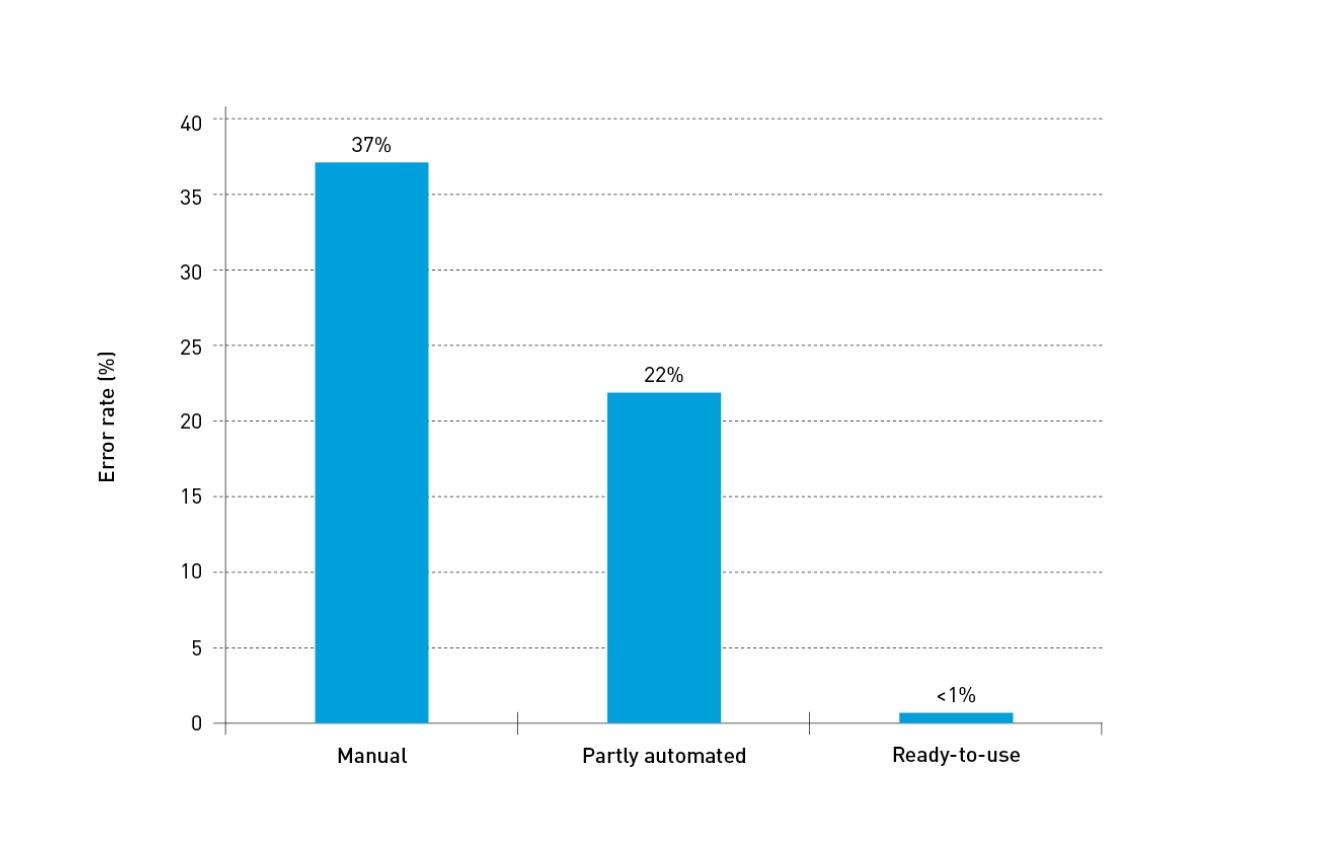
Current international guidelines recommend the use of standardised PN solutions in the majority of paediatric and newborn patients that require PN therapy.9 Standardised PN solutions help align nutritional intakes with guideline recommendations, helping to improve weight gain and avoid nutritional deficits.9 Commercial production of standard PN bags can assure better pharmaceutical control and better aseptic manufacturing conditions than the average hospital pharmacy.9 In an observational study to assess accuracy in compounding intravenous (IV) admixtures, commercially produced ready-to-use PN achieved an error rate of <1%.10
Error rate by type of compounded products10
Innovative nutritional therapy that meets the majority of needs

Supportive
Aligns with ESPGHAN/ESPEN/ESPR/CSPEN guidelines and supports growth and development in neonatal and paediatric patients.9

Secure
Commercially prepared, helping to lower the risk of medication errors and infections associated with custom compounding.9

Ready
Available for immediate nutritional support, with stability to store at room temperature for 18 months (Numeta G13%E Preterm/G16%E) and 24 months (Numeta G19%E).1-3
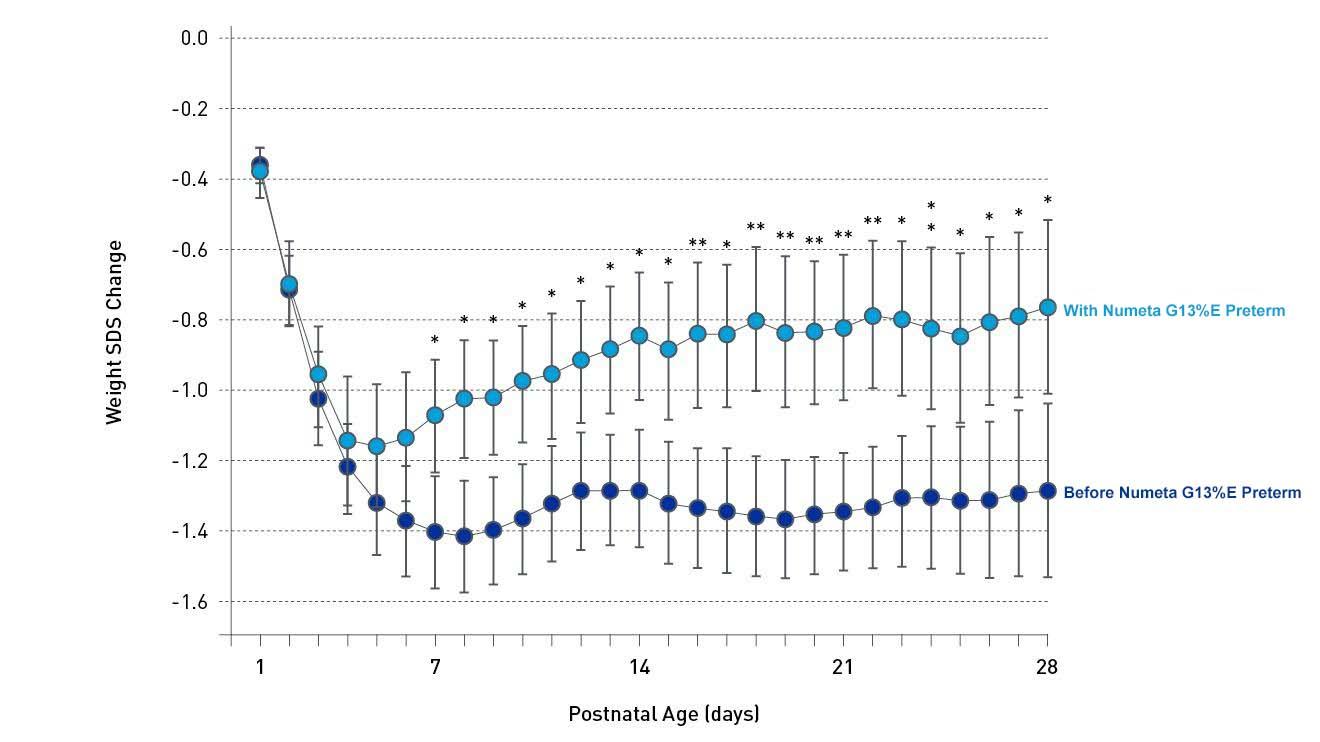
Numeta supports improved growth outcomes12
A retrospective study found that Numeta G13%E Preterm was associated with improved nutrient intakes and postnatal growth in very low-birthweight (VLBW) infants.12 The use of Numeta G13%E Preterm as a concentrated PN regime for VLBW infants showed improved weight and length gain during the first weeks of life with a persistent difference at 36 weeks post-menstrual age compared to infants given a pharmacy-prepared PN.12 A separate study in VLBW infants showed that Numeta G13%E Preterm was associated with improved protein intake and found a higher and faster likelihood of achieving protein targets.13
Numeta may help reduce hospital resource use and cost
According to a cost-consequences analysis, the increased use of ready-to-use 3CBs is associated with a decrease in adverse events and reduction in hospital labour, which can provide substantial resource and cost savings to the institution.5 These resources can be used to optimise hospital care in other areas. Across seven countries, there was an average of 3.4% savings to hospital budgets.5
Numeta provides a unique composition for delivering optimal nutrition

Numeta G13%E Preterm
Numeta G13%E Preterm is indicated for parenteral nutrition in preterm newborn infants when oral or enteral nutrition is not possible, insufficient or contraindicated.1

Numeta G16%E
Numeta G16%E is indicated for parenteral nutrition in term newborn infants and children up to 2 years when oral or enteral nutrition is not possible, insufficient or contraindicated.2

Numeta G19%E
Numeta G19%E is indicated for parenteral nutrition in children older than 2 years and adolescents 16-18 years old when oral or enteral nutrition is not possible, insufficient or contraindicated.3
Related Products
ADVERSE EVENT REPORTING
Adverse Events and any drug or medical device product quality complaints (including suspected defective medicines or medical device adverse incidents) should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse Events should also be reported to Baxter Healthcare Ltd, by email ([email protected]) or by phone (+44 (0)1635 206360). Drug or medical device product quality complaints relating to Baxter products can be reported directly to Baxter Healthcare Ltd by email ([email protected]) or by phone (+44 (0)1604 704603).